
About This Quiz
There are two types of people in the world - those who could talk about the periodic table all day, and those who hear the words and run! During this quiz, we're going to assume that you're the type of person who could talk science until your friends fall over from boredom. We're also going to put your brainy nature to the test and see how well you remember what you learned in chemistry class.
Created by Russian scientist Dmitri Mendeleev in 1869, the periodic table was originally based on the game of solitaire. As it was created, index cards with each element's information were arranged into suits - and the rest is history! While new elements have been found since its creation, Mendeleev knew enough to leave room for most of them. But how much do you remember about the periodic table?
Whether you passed the class with flying colors or this quiz will serve as your formal credit, we're curious to find out if you can get through all of our trivia questions without flaring up like magnesium when exposed to the air. Will you get as many of them right as you think you will, or will you need to take a remedial course? The elements await! Let's go find out!
Out of the 118 elements found on the periodic table, 90 of them can be found existing in nature. The other 28 elements are man-made, and they were inspired by technetium, the first man-made element.

After hydrogen (H), helium is the second most common element in the known universe. Tasteless, colorless and odorless, helium (He) is used for more than giving you a wacky voice at parties. It's one of the most unique elements on the periodic table.

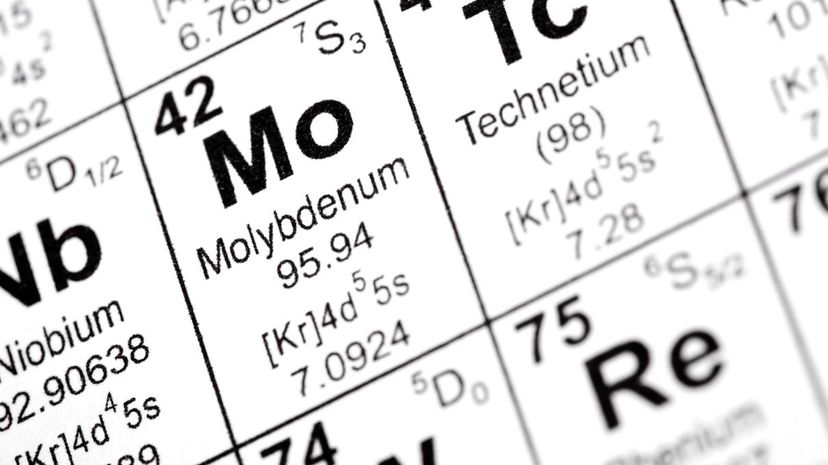
If you were working with the element denoted as "Fe" on the periodic table, you would be working with iron. Iron is the fourth most abundant element found in the earth's crust, and it's the sixth most common element in the entire universe.
Advertisement

We haven't yet been able to collect samples from Venus, Jupiter or Uranus, but we do know that the elements on Mars are identical to those found on Earth. Some of the elements found on Mars' crust are magnesium, iron and calcium.

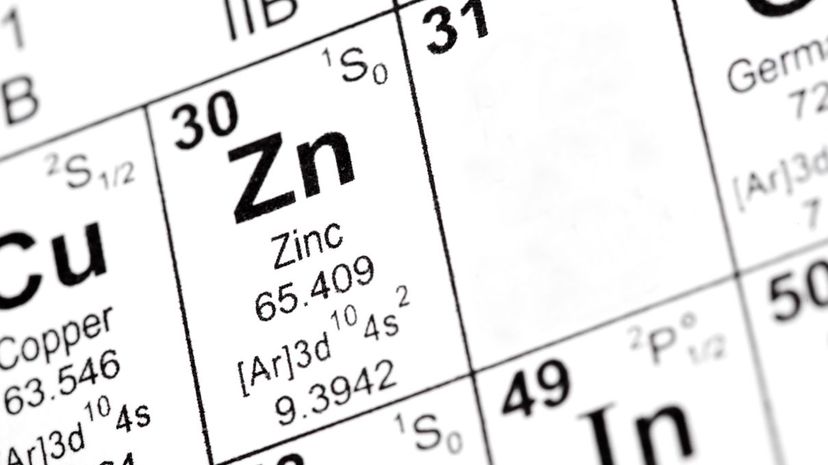
Milk does do a body good, and calcium in the reason. It's not often found in its pure form, but versions of it, like calcium carbonate, can be found in the oceans and many rocks, like limestone.

Nitrogen's atomic weight of 7 puts it right beside oxygen on the periodic table. When you breathe in, you inhale both elements, but nitrogen makes up the larger percentage.
Advertisement
When Mendeleev created the periodic table, he predicted that gallium - an element similar to aluminum- would soon be discovered. Only six years later, he was proven correct when a French scientist named Lecoq de Boisbaudran discovered it in 1875.

Gold, copper and silver are all part of Group 11 and casually called coin metals because of their use in making currency. Along with iron, osmium and hassium, iron belongs to Group 8.

A huge gaming enthusiast, University of St. Petersburg professor Dmitri Mendeleev decided to sort each element by its atomic weight. He then sorted them by "suit" with similar elements making up each column - just like the game of solitaire.
Advertisement

Every year, Argentina mines over 10 million pounds of silver. Known as The Land of Silver, it's the largest producer of the metal in the world. It only makes sense that it would have been named after the element Ag, or Argentum.

Symbolized as "U" on the periodic table, uranium is the heaviest element on earth. Its man-made counterpart, ununoctium, carries an atomic weight of 118. When the element with the atomic weight of 120 is discovered, ununoctium will have to take a back seat.

Both liquid and solid forms of oxygen appear as a pale blue, but it can come in other colors, too. It's the most abundant element in the human body, and it's the third most common element found in the entire universe.
Advertisement

When argon was discovered in 1894, Mendeleev decided to ignore it because it didn't fit into his version of the periodic table. Argon's name is taken from the Greek word "argos." Argos means lazy or idle, and the element's slow, nonreactive behavior makes it a natural fit.
When going from left to right on the periodic table, you'll notice that the number of valence electrons increases. Likewise, you'll notice that energy levels increase as you scan from top to bottom.

Found in the upper left-hand corner of the periodic table, you'll see the element named hydrogen. Denoted with the letter H, hydrogen makes up about 10% of the body of any living organism.
Advertisement

In countries like Norway and Poland, iodine is often called jod. You won't see the letter "J" on the periodic table, though. Iodine is still listed by its IUPAC symbol "I." "J" is the only letter of the alphabet left out.

Along with helium, xenon, radon and radon, these elements are called noble gases. The noble gases make up group 18 on the periodic table, and they're known for being relatively unreactive with other elements.

In 1869, a Russian chemist named Dmitri Mendeleev sorted the elements into the rows and columns that we know today. Using this method, he was able to predict the discovery of many new elements.
Advertisement
The part of an atom where the determining electrons are found is called the valence shell. The electrons found in the valence shell determine the chemical property and allow them to be classified as the correct element.
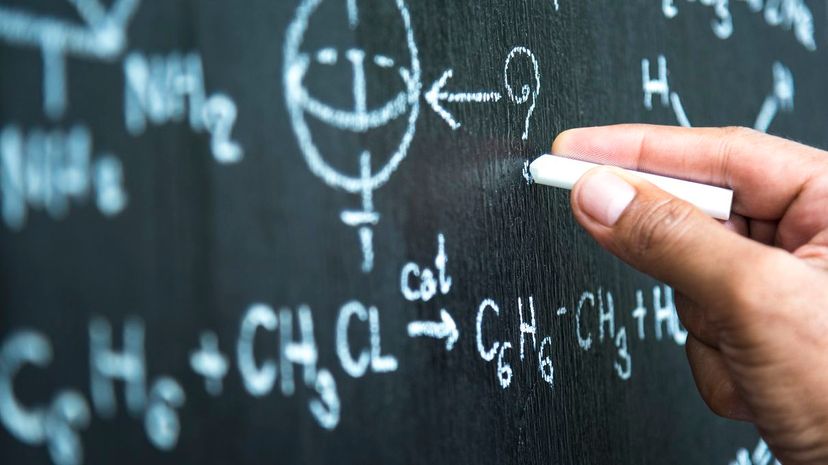
Usually represented by the symbol "Z," the atomic number identifies elements using the number of protons found in the nucleus. Sometimes called the proton number, it gives each element a unique signature.
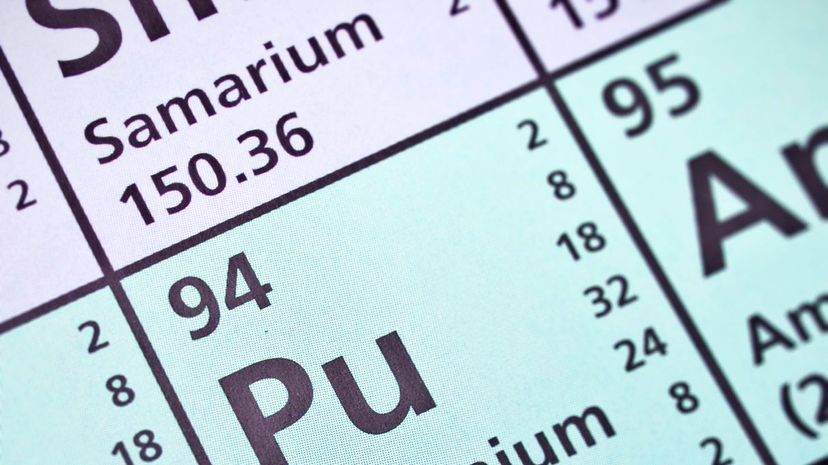
Francium has an atomic number of 87, and you can find it noted on the periodic table with the abbreviation "Fr." There are only a couple of ounces of it on earth, and it has no real use outside of scientific research.
Advertisement
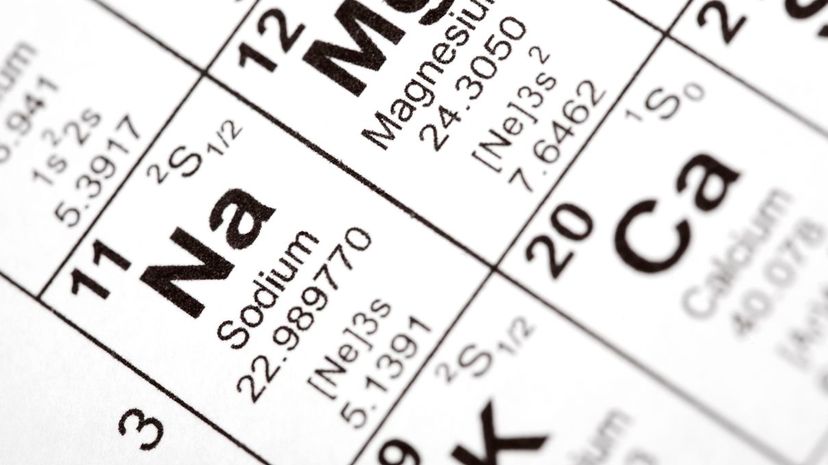
Each of the elements on the periodic table has four pieces of information: the atomic number, the element's symbol, the element's name and the mass number. The mass number, found at the bottom, indicates the number of protons and neutrons found in that element's nucleus.
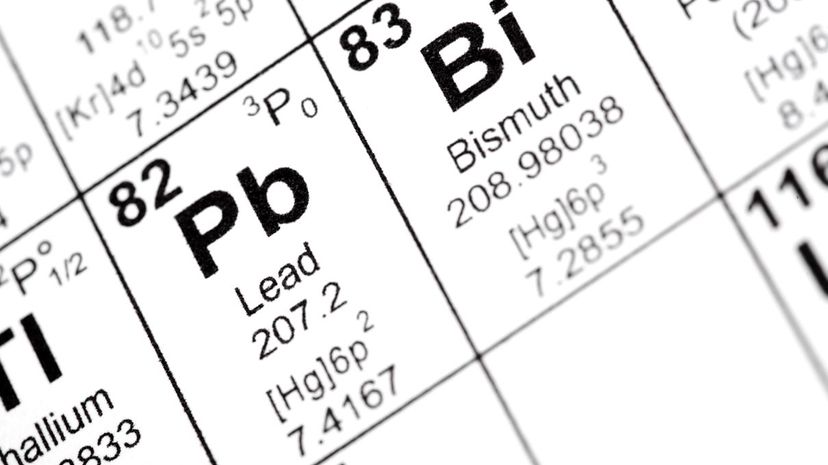
On the left side of the staircase, you will find the elements that belong to the metal family. To the right, you'll find metalloids that can perform as either metal or nonmetal elements.

Out of the 118 elements on the periodic table, at least 91 of them are metals. Wihile some of them appear in both the metallic and non-metallic categories, those that are not considered metals are called metalloids.
Advertisement

Both magnesium and phosphorus are considered highly reactive, but only phosphorus glows in the dark. Because phosphorus catches fire easily, it is often used on the tips of matches. It's phosphorus, not sulfur, that gives matches their stinky smell.
Found at the bottom left corner of the periodic table, the most active metals are alkali metals. The most reactive of them are helium, sodium and potassium - all of which react with water.

Elements with an atomic number of 92 or higher are created when parts of elements combine with other elements. Any atomic weight above that point is not considered a naturally occurring element.
Advertisement
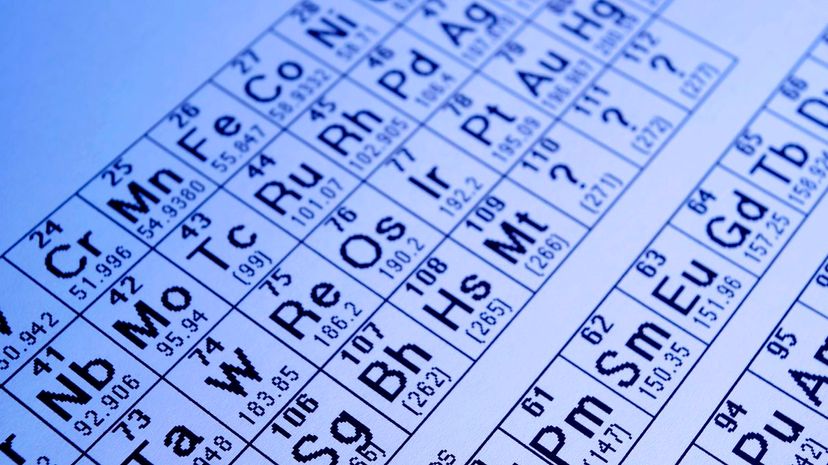
Simply put, each of the 18 columns in the periodic table is made up of similar elements. Each group - such as inert or noble gases - is put together so that chemists can more accurately predict how an element may react in different situations.

Carbon takes its name from "carbo," the Latin word for coal. Making up .032% of the earth's crust, carbon can be found in many forms, including diamonds, graphite and lithium-ion batteries.
Mendeleev assumed that all the elements were unchanging forms, but the study of radioactivity proved him wrong. Elements like uranium break down into other elements. Uranium's last breakdown is lead.
Advertisement

In their natural states, there are only two of the elements that are found in liquid form. Both mercury (Hg) and bromine (Br) are liquid at room temperature, but there are others that will melt when superheated.

When looking at the periodic table, you'll see a bunch of abbreviations that represent the elements. Some elements are easy to identify, but others, like gold, are a little harder. The correct abbreviation for it is Au - it's short for gold's Latin name, aurum.

The name "periodic table" comes from the horizontal rows that are called periods. Of the eight total periods, the first row has the least with only two elements. The sixth row has the most with 11.
Advertisement
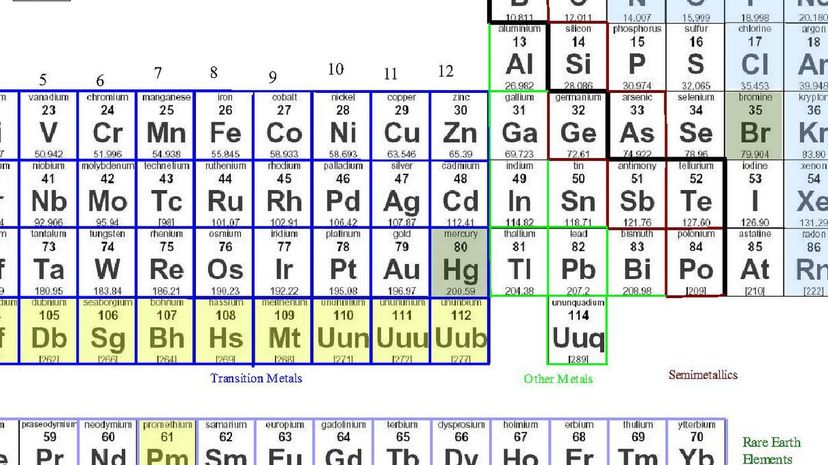
Over the course of history, the line that separates metal and nonmetal elements has been called many names. Most commonly called the staircase, you'll sometimes hear it called the amphoteric line and the metal-nonmetal line.

Because Dmitri Mendeleev was running out of time for a publishing deadline, he opted to assign atomic weights to each element. Rather than describing each element, giving each an atomic weight provided a shortcut for grouping them together.